Morphological and molecular diagnostic characters
reveal a new species of Pachyphytum (Crassulaceae)

Jerónimo Reyes Santiago1, Luis E. de La Cruz-López2*, Maria Kuzmina3, & Francisco Vergara-Silva1*
1 Jardín Botánico, Instituto de Biología, Universidad Nacional Autónoma de México. Circuito exterior s/n,Ciudad Universitaria, Copilco, Coyoacán, C.P. 04510, Ciudad de México, Mexico.
2 Posgrado en Ciencias Biológicas, Instituto de Biología, Universidad Nacional Autónoma de México. Av. Ciudad Universitaria 3000, C.P. 04360, Coyoacán, Ciudad de México, Mexico.
3 Centre for Biodiversity & Genomics, Biodiversity Institute of Ontario. University of Guelph, ON N1G 2W1, Ontario, Canada.
* Authors for correspondence: ledbiologia@hotmail.com; fvs@ib.unam.mx
Manuscript received 26th October 2018
Abstract: Pachyphytum viscidum (Crassulaceae: Saxifragales) is described as a new species to science. The specimens analyzed come from the Sierra de Santa Bárbara, Guanajuato, Mexico. Some morphological diagnostic characters for the new species are: sticky stems and leaves; dark green leaves; absence of wax on all their organs; and pale pink flowers. The new species is placed in the section Diotostemon of the genus, with possible proximity to P. brevifolium and P. hookeri. DNA sequence analysis from the matK and rbcL chloroplast regions, as well as ITS2 nuclear loci, revealed the presence of molecular diagnostic sites which, in addition to the aforementioned morphological character states, further support the taxonomic description as well as future integrative taxonomy studies in Mexican Crassulaceae.
Resumen: Se describe a Pachyphytum viscidum (Crassulaceae: Saxifragales) como una especie nueva para la ciencia. Los especímenes analizados provienen de la Sierra de Santa Bárbara, Guanajuato, México. Algunos caracteres morfológicos diagnósticos para esta especie son: sus tallos y hojas glutinosas; hojas verde oscuro; la ausencia de cera en sus órganos; y el color rosa tenue de sus flores. Se le ubica dentro de la sección Diotostemon, en posible cercanía con P. brevifolium y P. hookeri. El análisis de secuencias de ADN de las regiones del cloroplasto matK y rbcL, así como del locus nuclear ITS2, reveló la presencia de sitios diagnósticos moleculares que, en adición a los morfológicos, también apoyan la descripción taxonómica así como estudios futuros de taxonomía integrativa en Crassulaceae de México.
Keywords: Crassulaceae, character-based DNA barcoding, integrative taxonomy, ITS2, matK, Pachyphytum
INTRODUCTION
The genus Pachyphytum (Crassulaceae) was proposed by Link, Klotzsch et Otto (Klotzsch, 1841) with P. bracteosum Klotzsch (Moran, 1989) as the type species. Moran (1968) recognized three sections based on morphological affinities: Pachyphytum, Diotostemon and Ixiocaulon. Later, J. Thiede (in Eggli, 2003) merged the species of sect. Ixiocaulon into sect. Pachyphytum, resulting in only two sections: Diotostemon and Pachyphytum. Genetic distance-based classification of the genus employing AFLP molecular markers suggested two groups inside Pachyphytum (García-Ruíz 2003). In this classification, group A included species P. bracteosum, P. brevifolium, P. glutinicaule, P. hookeri, P. longifolium, and P. oviferum; while group B incorporated P. caesium, P. coeruleum, P. compactum, P. contrerasii, P. fittkaui, P. garciae, P.kimnachii, P. machucae, P. rzedowskii, P. viride, and P. werdermannii. It should be noted that neither Moran´s nor Thiede´s groupings match García Ruíz´s results.
A recent molecular phylogenetic analysis of the genus Echeveria and related genera including Pachy-phytum, based on a combined DNA matrix of rbcL, matK and ITS2 genomic regions, retrieved the latter genus as a monophyletic group (Cruz-López etal., 2019) sister to the rest of Cremnophila, Echeveria, Graptopetalum, and some Sedum and Thompson-ella species. Another significant result of this work indicated that Pachyphytum cuicatecanum (J.Reyes, Joel Pérez & Brachet) Kimnach is closely related to several Echeveria species (E. penduliflora and others) and clearly do not belong to Pachyphytum, despite sharing many morphological character states. Nevertheless, low resolution problems do not allow the identification of groups (sections) inside Pachy-phytum. Previous studies on the “Echeveria Group” (Carrillo-Reyes et al., 2009; Vázquez-Cotero et al., 2017) also recovered Pachyphytum as a monophyletic2017) also recovered Pachyphytum as a monophyletic group, resting on partial species sampling. Historically, the placement of taxa in infrageneric categories (sections) has been complicated, because there is often little congruence between morphological, biogeographic and DNA datasets; this discrepancy between different sources of evidence may have contrasting explanations (e.g. recent diversification vs. decoupling between molecular and morphological evolution).
The most outstanding character states of Pachyphytum are: presence of membranous scale-like structures known as appendages on the inside of everypetal, one on each side of the epipetalous filaments although, some Echeveria species also possess appendages (E. heterosepala, E. longissima var. longissima, E. dactylifera, E. purhepecha, E. rulfiana, among many others); pronounced succulence of the leaves; and pendant scorpioid cymose inflorescences (Reyes et al., 2007). At present, the genus consists of 24 described species — based on morphological, phenological, ecological and geographical distribution aspects — all endemic to Mexico and distributed mainly in central and western regions of this country.
In 2003, a collection of living plants of the family Crassulaceae (Saxifragales: Rosidae) was started at the Jardín Botánico, Instituto de Biología, Universidad Nacional Autónoma de México. In 2005, the Mexican Association of Botanical Gardens (Asociación Mexicana de Jardines Botánicos) declared this collection as “Colección Nacional” (National Collection), representing 70% of the Crassulaceae species known in Mexico. For the establishment of this national collection program, botanical explorations were necessary, as well as the cultivation andmaintenance of species of different genera with a georeferenced database. During fieldwork, the genus Pachyphytum received primary attention. The work with the collection has received a great impulse from Christian Brachet, member of the Mexican Cactus Society (Sociedad Mexicana de Cactología, A.C.), who has provided significant help with the logistics of the explorations and with finding information about the type localities of the known species.
The present paper derives from the “Crassulaceae of Mexico” (“Crasuláceas de México”) long-term research project, based on the National Crassulaceae Collection and hosted by JB-IBUNAM. A primary aim of this project has been the gradual construction of a DNA barcoding reference library for Mexican Crassulaceae, to be linked with other datasets in an integrative taxonomy (DeSalle et al., 2005; Goldstein and DeSalle, 2011) framework. Character based methods, such as CAOS (Sarkar et al., 2008), have been given preference to establish DNA barcodes, as they allow the definition of discrete DNA diagnostics compatible with apomorphic, morphological character states.
The species described here was first located near La Presita at the base of the Sierra de Santa Bárbara, Guanajuato, in May of 2004 by the team of Christian Brachet, Joel Pérez Crisanto, Roxana Mondragón, and Jerónimo Reyes Santiago. The discovery was made during a trip to locate populations of Echeveria calderoniae Perez-Calix growing in the same mountains. At the time of collection, the plants were flowering. The exact locality where the specimens were found is a ravine with high amounts of humidity during the rainy season. Recently, Julia Etter and Martin Kristen have sent specimens of Pachyphytum sp. with identical morphological characteristics from the states of Aguascalientes, Jalisco and Zacatecas to the JB-IBUNAM, and have also shared excellent photos of the new species in habitat.
MATERIALS AND METHODS
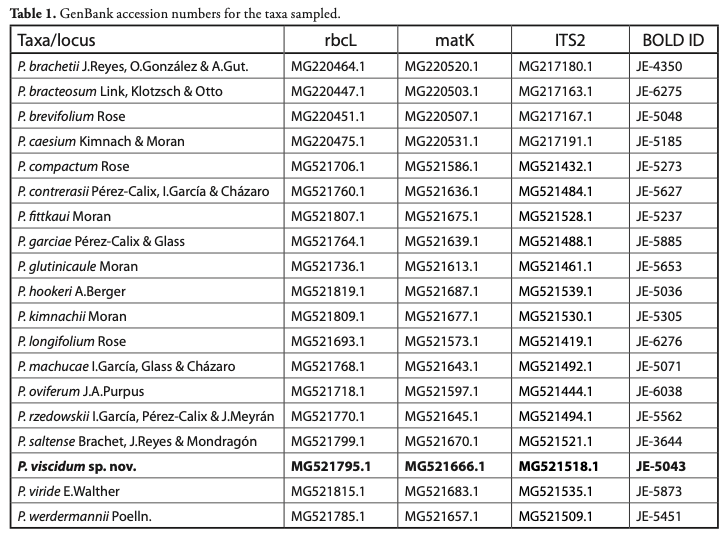
Sampled taxa: 19 known species of Pachyphytum were sampled, including the new species (Table1). Five species were not sampled: P. coeruleum J. Meyrán, P. confusum Pérez-Calix, Guadián-Marín & I. García, P. cuicatecanum (J. Reyes, Joel Pérez & Brachet) Kimnach, P. neglectum and P. rogeliocardenasii Pérez-Calix & R. Torres. Regarding P. cuicatecanum, an independent phylogenetic analysis indicates that it does not belong to Pachyphytum (Cruz-López et al., 2019). Due to their recent description, materials were not available for P. coeruleum, P. confusum, P. neglectum, and P. rogeliocardenasii. At least one specimen of the sampled Pachyphytum species was used in molecular analysis.
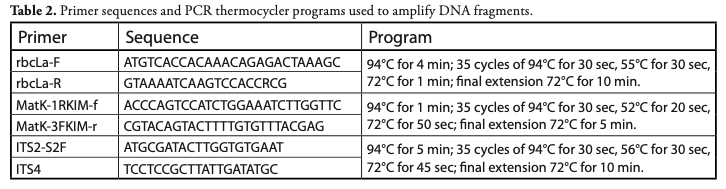
DNA extraction, amplification and sequencing: Total DNA was extracted from silica-gel dried tissue using the 2X CTAB protocol [standard method at the Canadian Centre for DNA Barcoding (CCDB, University of Guelph, Ontario, Canada)]. DNA fragment amplifications were carried out using Platinum® Taq DNA Polymerase (Ivanova et al., 2006). Pairs of primers for the three regions selected for amplification and the PCR thermocycler programs used are summarized in Table 2, according to CCDB standards. For rbcL and ITS2 sequences, amplification was performed in 12.5 µL final volumes with 6.25 µL of 10% trehalose. For matK, 1.875 µL of 20% trehalose was used. PCR products were visualized in pre-cast agarose E-gel® 96 units from Invitrogen. DNA fragments were sequenced at the CCDB facilities. Contig assembly and sequence edition was carried out in Geneious v. R9 (http://www.geneious.com; Kearse et al., 2012) and submitted to BOLD Systems (Barcode Of Life Database, http://www.boldsystems.org/). Accession numbers and BOLD Systems IDs for all sequences are provided in Table 1
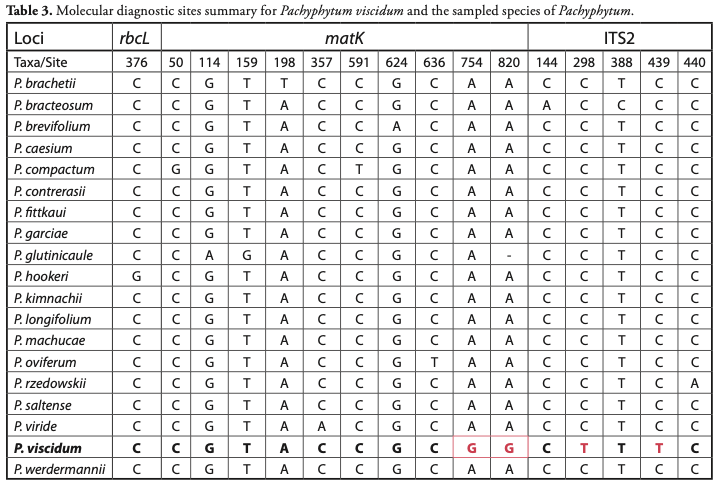
Molecular diagnostics analysis: Aligned DNA sequences for the selected markers were downloaded from BOLD Systems. In the alignments, each sequence per species was inspected with PhyDE v0.9971 (http://www.phyde.de/) and/or analyzed with CAOS (Sarkar et al., 2008), in order to identify DNA diagnostic character states in homologous nucleotide sites per species. These molecular diagnostics were selected and summarized (in order) in Table 3.
RESULTS
Pachyphytum viscidum Reyes & de la Cruz-López, sp. nov. (Figs. 1 & 2).
Type: — Mexico. Guanajuato: Municipality Ocampo, Las Presitas, Santa Bárbara, near the base of Sierra de Santa Bárbara, altitude 2200m. Jerónimo Reyes Santiago & C. Brachet 5043 (Holotype: MEXU).
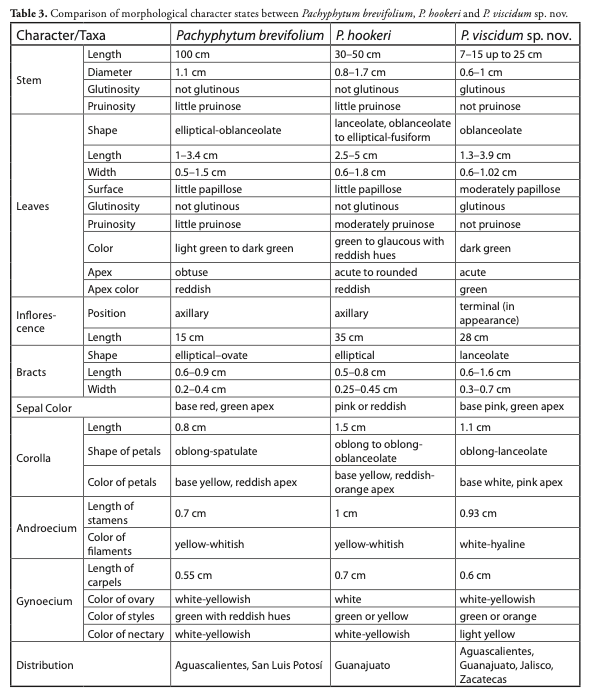
Pachyphytum viscidum is morphologically related to P. brevifolium and P. hookeri, but differs by having dark green sticky leaves, the absence of wax on all its organs, and pale pink flowers.
Plant perennial, suffrutescent, caespitose. Roots fibrous. Stems erect, 7–15 cm tall (up to 25 cm in cultivation), 0.6–1.0 cm thick, brownish to ochre green, glutinous, with fissures toward the base, branched from the base. Rosettes lax, not well defined, 3–6 cm in diameter. Leaves oblanceolate, subcylindrical, ascending, sometimes extended, 1.3–3.9 cm long, 0.6–1.2 cm wide, 0.5–0.72 cm thick, apex mucronulate, slightly acute, with even margins, slightly concave, dark green and glossy. Inflorescences 21.5–28 cm long, 0.3–0.45 cm thick at the base of the peduncle, with ascending cincinni almost 6 cm long, later pendant, faint pink colored with 4–10 flowers. Bracts 10–12, lanceolate, 0.6–1.6 cm long, 0.3–0.7 cm wide, the ones at the base small, pinkish to light green. Pedicels 0.6–1.1 cm long, 0.15–0.2 cm thick, smooth, faint pink. Calyx 0.79–0.86 cm long, 0.78–0.8 cm in diameter, sepals 5, almost equal, lanceolate, 0.78–0.85 cm long, 0.29–0.43 cm wide, pink, green towards the tip, apex acute, mucronulate. Corolla tubular, slightly campanulate, 1.1 cm long, 0.7 cm wide. Petals 5, free from the base, 1.09–1.14 cm long, 0.38–0.43 cm wide, oblong to lanceolate, base slightly hyaline to whitish from the middle to the tip, faint pink on both sides, apex mucronulate, appendages hyaline with an irregular tip.
Androecium: epipetalous stamens 5, 0.6–0.62 cm long, antisepalous stamens 5, 0.85–0.93 cm long, hyaline, anthers yellow in anthesis. Nectaries reniform, yellowish, almost 0.2 cm wide. Gynoecium: carpels 5, 0.52–0.6 cm long, yellowish, style 0.2 cm long, greenish to orange colored, stigmas greenish. Fruits brownish polyfollicles with many oblong brownish seeds.
Distribution and habitat: Four localities are currently known in the Mexican highlands (Aguascalientes, Guanajuato, Jalisco and Zacatecas) in Quercus forests in ravines surrounded by Pinus cembroides Zucc. Associated species are Echeveria agavoides Lem., Yucca filifera Chabaud, Ferocactus histrix (DC.) G.E. Linds., Mammillaria spp., Beschorneria sp., Juniperus sp., among others.
Phenology: Flowers from January to May.
Etymology: The specific epithet refers to the viscidity (glutinosity) of the stems and in particular the young leaves of the species.
DISCUSSION
Although phylogenetic relationships in Pachyphytum are not clearly established, P. viscidum is placed in the sect. Diotostemon (Salm-Dyck) E. Walther based on morphological affinities. In terms of overall appearance, the collected plants are similar to P. hookeri, a species abundant in the area. Furthermore, they are also related to P. brevifolium which also occurs in the state of Guanajuato. However, on closer inspection P. viscidum is distinguished from the two latter ones because its leaves and stems are glutinous, and by the absence of wax (farina) on the leaves.
Furthermore, the plants are dark green colored, and the inflorescence born very close to the apical meristem giving the impression of a terminal inflorescence. The new species shows similarities to P. hookeri (Salm-Dyck) A. Berger (Moran, 1990) regarding the shape and coloration of sepals and petals (Fig. 3), but differs by its sticky stems and leaves, dark green thin leaves without wax (Table 4). P. viscidum can also be related to P. brevifolium Rose (Moran, 1963; Pérez-Calix & Glass, 1999) because of its oblanceolate leaves, it is however differentiated from the latterin having longer flowers with narrowed sepals as well as pale pinkish petals and green styles and stigmas
(Fig. 3). In short, twenty-eight apomorphies were found among the taxa analyzed (Table 4).
Pachyphytum viscidum is one of the species in the genus Pachyphytum that shows molecular diagnostic characters in accordance with character-based DNA barcoding. Referencing the alignment generated in the Barcode of Life Database (BOLD systems), diagnostic characters for P. viscidum correspond to posi-tions 754 and 820 of matK with a G in contrast to the A of P. brevifolium and P. hookeri and the rest of the Pachyphytum species; in turn for region two of the intergenic spacer (ITS2), P. viscidum can also be differentiated from all Pachyphytum species sampled by two DNA diagnostics in positions 298 and 439, both with the T nucleotide, in contrast to the presence of C in the rest of the sequences of all otherPachyphytum species (Table 3).
The description of Pachyphytum viscidum is further supported under integrative taxonomy (sensu DeSalle et al., 2005) criteria: there are both morphological and molecular diagnostic character states associated with the specimens analyzed. Regarding the two genomic regions where DNA diagnostics were found for the new Pachyphytum species, they are currently included in the group of revised core and supplementary loci to perform DNA barcoding in plants (CBOLPlant Working Group, 2009; Hollingsworth, 2011).
The use of these two sources of characters as a whole for the description of P. viscidum is enabled by theoperational diagram of integrative taxonomy, called “taxonomic circle” by DeSalle et al. (2005; see also Goldstein & DeSalle, 2011). According to the taxonomic circle’s different options proposed by DeSalle et al. (2005), the case of P. viscidum corresponds with “sympatric species” where the combination of morpho logical and molecular evidence supports a new species status for the collected specimens, although they cooccur geographically with other species.”



Additional specimens examined: P. brachetii. Mexico. Hidalgo: Mpio. Actopan, 5 km al sur de Actopan, J. Reyes 4350 (MEXU). P. bracteosum. Mexico. Hidalgo: Laguna de Meztitlán, J. Reyes 6275 (MEXU). P. brevifolium. Mexico. Guanajuato: Los Picones, J. Reyes 5048 (MEXU). P. caesium. Mexico. Aguascalientes: Barranca Montoro, J. Reyes 5185 (MEXU). P. compactum. Mexico. Querétaro: La Cañada, J. Reyes 5273 (MEXU). P. contrerasii. Mexico. Jalisco: Mirador Dr. Atl, J. Reyes 5627 (MEXU). P. fittkaui. Mexico. Guanajuato: Los Jofres, J. Reyes 5237 (MEXU). P. garciae. Mexico. Querétaro: El Zapote, J. Reyes 5885 (MEXU). P. glutinicaule. Mexico. Hidalgo: Mpio. Tasquillo, Puerto Juárez, J. Reyes 5653 (MEXU). P. hookeri. Mexico. Guanajuato: Cerro Gordo, J. Reyes 5036 (MEXU). P. kimnachii. Mexico. San Luis Potosí: Cerro Agujón, J. Reyes 5305 (MEXU). P. longifolium. Mexico. Hidalgo: entre Gilo y Almolon, J. Reyes 6276 (MEXU). P. machucae. Mexico. Michoacán: Cañada de Agua, J. Reyes 5071 (MEXU). P. oviferum. Mexico. San Luis Potosí: El Cajón, J. Reyes 6038 (MEXU). P. rzedowskii. Mexico. Michoacán: 1.5 km al noroeste de Puente Grande, J. Reyes 5562 (MEXU). P. saltense. Mexico. Zacatecas: El Salto, J. Reyes 3644 (MEXU). P. viscidum. (paratypes).Mexico. Aguascalientes: Mpio. Tepezala, in cliffs above RMO Tepezala, 2421 m, June 16, 2012, Julia Etter & Martin Kristen 3619 (MEXU). Jalisco: Mpio. Ojuelos de Jalisco, 3 km NW of Matanzas, between Lagos de Moreno and Ojuelos along highway 80, in cliffs behind the cemetery, 2320 m, March 27, 2012 (flowering), Julia Etter & Martin Kristen 3494 (MEXU). Zacatecas: Mpio. Villa García, 4.5 km from Villa García along dirt road to Loreto, 2150 m, May 15, 2012, Julia Etter & Martin Kristen 3531 (MEXU). P. viride. Mexico. Guanajuato: entrada al Pueblo de Coconoxtle, J. Reyes 5873(MEXU). P. werdermannii. Mexico. Tamaulipas: Microondas Las Mulas, Julia Etter & Martin Kristen 2000.
ACKNOWLEDGMENTS
Financial support for this work was provided by UNAM-DGAPA-PAPIIT [Project IN212015, “Taxonomía integrativa y códigos de barras genéticos en Echeveria (Crassulaceae)” awarded to F. V.-S.] and CONACYT [Project 247078, “Iniciativa interdisciplinaria para el aprovechamiento sustentable del género Echeveria (Crassulaceae), plantas con potencial hortícola para la producción en áreas rurales de México” awarded to F. V.-S.] as well as Project [LA-NABIO: 232746 “Laboratorio Nacional de Biodiversidad”]. The first author acknowledges reception of graduate studies scholarships from Posgrado en Ciencias Biológicas, UNAM and CONACYT. We also thank Julia Etter and Martin Kristen for the plant material provided. Special thanks to the anonymous reviewers for the valuable comments that improved the paper.
LITERATURE CITED
Carrillo-Reyes, P., Sosa, V. & Mort, M. E. 2009. Molecular phylogeny of the Acre clade (Crassulaceae): Dealing with the lack of definitions for Echeveria and Sedum.
Molecular Phylogenetics and Evolution 53: 267–276.
CBOL Plant Working Group. 2009. A DNA barcode for land plants. Proceedings of the National Academy of Sciences USA 106: 12794–12797.
De la Cruz-López L. E., Vergara-Silva F., Reyes Santiago
J., Espino Ortega G., Carrillo-Reyes P. & Kuzmina M.
2019. Phylogenetic relationships of Echeveria (Crassulaceae) and related genera from Mexico, based on three DNA barcoding loci. Phytotaxa 423: 33–57.
DeSalle R, Egan, M. G. & Siddall, M. 2005. The unholy trinity: taxonomy, species delimitation and DNA barcoding. Philosophical Transactions of the Royal Society B 360: 1905–1916.
García R, I. 2003. Relaciones interespecíficas del género Pachyphytum (Crassulaceae), empleando marcadores genéticos AFLP. M.Sc. Thesis. Facultad de Ciencias Biológicas y Agropecuarias. Universidad de Colima.
Goldstein, P. Z. & DeSalle, R. 2011. Integrating DNA barcode data and taxonomic practice: determination, discovery, and description. Bioessays 33: 135–147.
Hollingsworth, P. M. 2011. Refining the DNA barcode for land plants. Proceedings of the National Academy of Sciences USA 108: 19451–19452.
Ivanova, N.V., Dewaard, J. R & Hebert, P. D. N. 2006.
An inexpensive, automation-friendly protocol for recovering high-quality DNA. Molecular Ecology Notes 6: 998–1002.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S.,
Cheung, M., Sturrock, S., Buxton, S., Cooper, A.,
Markowitz, S., Duran, C., Thierer, T., Ashton, B.,
Mentjies, P. & Drummond, A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649.
Klotzsch, J. F. 1841. Einer neuen mexikanischen Pflanze Pachyphytum bracteosum. In: Otto, F. & Dietrich, A.G. (Eds.) Allgemeine Gartenzeitung 9: 9.
Moran, R. 1963. Pachyphytum brevifolium Rose and P. glutinicaule, a new species from Hidalgo, Mexico. Cactus and Succulent Journal of America 35: 35–41.
Moran, R. 1968. New subgeneric groups in Echeveria and Pachyphytum. Cactus and Succulent Journal of America 40: 36–42.
Moran, R. 1989. Pachyphytum bracteosum Klotzsch. Cactus and Succulent Journal of America 61: 119–124.
Moran, R. 1990. Pachyphytum hookeri (Salm-Dyck),
Berger (Crassulaceae). Cactus and Succulent Journal of America 62: 236–241.
Perez-Calix, E., & Glass, C. 1999. Pachyphytum brevifolium Rose (Crassulaceae) a un siglo de su descubrimien-
to y Pachyphytum garciae una especie nueva del centro de México. Acta Botánica Mexicana 48: 1–10.
Reyes S, J., González Z, O., Gutiérrez R, A., 2007. Pachyphytum brachetii, una nueva especie del estado de Hi-
dalgo, México. Cactáceas y Suculentas Mexicanas 52: 53–63.
Sarkar, I. N., Planet, P. J. & DeSalle, R. 2008. CAOS software for use in character-based DNA barcoding.
Molecular Ecology Resources 8: 1256–1259.
Thiede, J. 2003. Pachyphytum. In: Eggli, U. (ed.). Crassulaceae. Illustrated handbook of succulent plants. Spring-
er. Berlin, Germany. pp. 190–195.
Vázquez-Cotero, C., Sosa, V., & Carrillo-Reyes, P. 2017.
Phylogenetic position of Echeveria heterosepala(Crassulaceae): a rare species with diagnostic characters of Pachyphytum. Botanical Sciences 95: 515–526.
